A review by Dr Deepak Chitkara from Birla Institute of Technology and Science, Pilani, Rajasthan; Drs Vivek Singh and Indumathi Mariappan from L V Prasad Eye Institute and others looks at genome editing using CRISPR/Cas9 as a potential therapeutic strategy to treat retinal dystrophies. The review explores the applicability of non-viral nanomedicine for such treatments.
Retinal dystrophies are progressive blinding conditions that affect the retina of the eye. They are of diverse origin and most inherited forms of this disease do not have effective treatments. It is estimated that about 1 in 2000 people world over develop inherited retinal dystrophies (IRDs). The unique and closed nature of the retinal environment makes it a complex region for treatment. Vitreoretinal specialists administer intravitreal drug injections to manage symptoms of some retinal dystrophies that involve abnormal blood vessel growth. However, such treatments need multiple interventions and can only address the symptoms but not the cause, which in the case of IRDs, are the inherited defects in the genes of retinal cells.
Today, gene supplementation therapies offer the promise of tackling such genetic defects. The CRISPR/Cas based gene editing tools in particular are at the forefront of new gene therapeutics for various inherited genetic disorders. This revolutionary system can make precise and controlled edits to specific mutation sites within the genes inside a cell. ‘The retina is particularly suited for such therapies thanks to the eye’s immune-privileged status, the presence of the blood-retina barrier, the non-proliferative nature of retinal cells and the involvement of single gene mutations in most IRDs (monogenic diseases)’, says Dr. Indumathi Mariappan, Research Scientist at the Center for Ocular Regeneration, LVPEI. A recent LVPEI report has also reviewed CRISPR-based therapy for inherited corneal diseases (ICDs). However, the use of CRISPR/Cas system as a therapeutic drug is still early in its evolution. The system today has several issues such as low editing efficiency, off-target edits and unwarranted genomic alterations. The delivery mechanisms and new vectors to deliver the CRISPR/Cas system continue to be developed and the therapeutic environment is complex and promising.
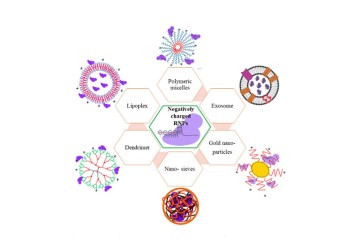
A review paper in the Asian Journal of Pharmaceutical Sciences by Drs Deepak Chitkara, Vivek Singh, Indumathi Mariappan and others presents an overview of different delivery strategies for the CRISPR/Cas system to treat retinal dystrophies. The paper discusses ongoing clinical trials for gene therapies that are targeting a variety of retinal dystrophies. It highlights the promise of developing one-time treatments and the risk of undesirable off-target effects. The paper describes the three main approaches of CRISPR delivery: plasmid DNA, mRNA, or ribonucleoprotein (RNP) complexes. It also highlights several challenges in achieving the desired gene-edit, including the problems with the use of viral vectors for CRISPR-Cas delivery.
The authors also explored in detail a new range of non-viral delivery systems such as, lipids and synthetic polymeric complexes, that have the potential to efficiently deliver CRISPR cargos in the form of DNA or RNA or as RNP complexes into target cells. The paper showcases the promise of these newer tools for delivering copies of a normal gene, or CRISPR tools for ‘editing’ a mutation in the affected gene so that, it can restore normal protein expression.
‘The CRISPR/Cas system is a promising and innovative technology to treat a host of inherited ocular dystrophies; not just retinal dystrophies but also corneal dystrophies like CHED, FECD and more,’ says Dr. Vivek Singh. 'However, there are some limitations like offsite mutation, efficacy and safety which need to be overcome with better research to be widely accepted as standard of care for these conditions.'
Dr Vivek Singh acknowledges the role of graduate students Mohammad Salman and Deepak Sahel for exploring the feasibility of non-viral delivery systems discussed in this paper.
Citation
Lohia A, Sahel DK, Salman M, Singh V, Mariappan I, Mittal A, Chitkara D. Delivery strategies for CRISPR/Cas genome editing tool for retinal dystrophies: challenges and opportunities. Asian J Pharm Sci. 2022 Mar;17(2):153-176. doi: 10.1016/j.ajps.2022.02.001. Epub 2022 Feb 13. PMID: 36320315; PMCID: PMC9614410.
Photo credit: Non-viral vectors being explored for the delivery of CRISPR/Cas RNPs; Lohia et al.