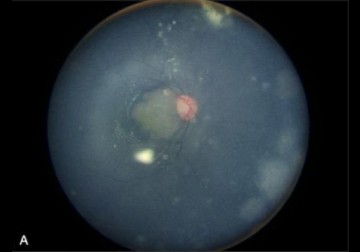
In a new study from LVPEI, Vijitha Vempurulu, Vishal Raval and Swathi Kaliki evaluate the safety and efficiency of high dose topotecan monotherapy in Indian retinoblastoma patients.
Young children can develop a rare, life-threatening eye cancer called retinoblastoma that is identified as a ‘white spot’ in the eye. If detected and treated early, the chances of preserving the child’s eye and saving their life are high. A key innovation has been the introduction of intravenous chemotherapy, which has greatly improved eye preservation rates and saved the lives of these children. In some cases, however, the tumor returns (recurrence) or does not respond to treatment (refractory). In such cases, intravitreal chemotherapy, where the drug is injected into the gel-like cavity (vitreous cavity) of the eye, has emerged as an alternative to control the tumor, with a better chance of preserving the eye.
Melphalan has been the most widely used intravitreal drug, but it has a risk of damaging the retina at high doses. Studies show that Topotecan at higher doses (above 90 mcg, instead of previously unsuccessful preclinical doses of 20-30 mcg) might work well without damaging the retina and hence it has been explored as a safer alternative. However, there have been no reports of its use in the Indian population. The Asia-Pacific region carries the highest burden of retinoblastoma cases worldwide, with India reporting the largest number of cases. Treatment responses and side-effects can differ across populations making it important to study outcomes in our population too before making Topotecan a standard of care for refractory or recurrent retinoblastoma.
In a new study published in the journal Retina, Drs. Vijitha. S. Vempurulu, Vishal Raval, and Swathi Kaliki from LVPEI analyzed seven Indian patients with different forms of retinoblastoma who received high-dose intravitreal topotecan IVitT100 (100 µg). Of the seven (5 male and 2 female), two patients had refractory tumors while five had recurrent tumors, and all tumors had grown beyond the original locus. Six patients received intravenous chemotherapy, while one patient received intra-arterial chemotherapy, among other treatments. After a mean interval of 9 months, intra-vitreal Topotecan treatment was initiated in these patients.
The treatment was effective in controlling tumour in five out of seven eyes (71%), with complete tumour control (100%) seen in three different types of retinoblastomas. In the two patients with refractory tumor, an alternate treatment was also provided. However, one patient had a relapse after five months and was then successfully treated with cryotherapy. All seven eyes were saved (100%), no drug related side-effects were noted, and the retina continued to function normally in all four patients who were tested with electroretinography, a procedure that measures the electrical activity of the retina in response to light. The findings suggest that high-dose intravitreal topotecan could be a stand-alone treatment in selective cases with recurrent or refractory retinoblastoma.
‘High-dose intravitreal topotecan holds great promise for refractory cases of retinoblastoma and may be a cost-effective alternative to intra-arterial chemotherapy’, comments Dr. Swathi Kaliki, head of the Operation Eyesight Universal Institute for Eye Cancer at LVPEI.
Citation:
Vempuluru VS, Raval V, Kaliki S. High-dose intravitreal topotecan (100 mcg/0.1cc) as monotherapy for recurrent/refractory intraocular retinoblastoma in seven eyes. Retina. 2025 Jul 3. doi: 10.1097/IAE.0000000000004601.
Photo credit: Fig. 1, Vempuluru et al.